Stoichiometry of Maltose
Fermentation to Ethanol
©
James
M. Gossett (March 25, 2012) Questions/Comments?
![]()
![]() Overview
Overview
![]() Stoichiometry of Ethanol Production From Maltose
Stoichiometry of Ethanol Production From Maltose
![]() Contribution of Ethanol to Apparent Brix
Contribution of Ethanol to Apparent Brix
![]() Measurement of %ABV Using Brix Refractometry
Measurement of %ABV Using Brix Refractometry
![]() Derivation of Calculator Equations
Derivation of Calculator Equations
![]() Evaluation of The Method: Comparing Estimated and Measured %ABV
Evaluation of The Method: Comparing Estimated and Measured %ABV
Consideration
of two factors is required to estimate ethanol (EtOH)
production from initial and final refractometer
(Brix) measurements:
(i)
the stoichiometry of maltose fermentation to EtOH; and
(ii)
the contribution that the resulting EtOH makes to Brix.
In this
section, the stoichiometry is considered.
Wort is a complex
mixture of carbohydrates, but I will simplify by assuming that the major
fermentable fraction is maltose, C12H22O11 (FW
= 342 g/mol). Carbohydrate composition certainly
varies among beer-types, but maltose is by far the sugar at highest
concentration in most worts, constituting about 50%
of wort carbohydrate.10
Given that wort carbohydrate is only
about 65% to 75% fermentable (and maltose is 100% fermentable), then maltose
certainly constitutes far more than 50% of the fermentable carbohydrate
fraction of wort. The next highest sugar fractions are
glucose and maltotriose, which are generally each
present at only about 1/5th the levels of maltose. [See Table 2.3 in Boulton
and Quain.10] Since maltose (a
disaccharide) has a chain-length midway between glucose (a monosaccharide) and maltotriose (a trisaccharide),
that also makes it a particularly good stand-in for the fermentable extract of wort.
Balling’s Equation
Carl J. N. Balling1,2 observed
the following stoichiometry:
2.0665 g
fermentable extract ---> 1 g EtOH + 0.9565 g CO2
+ 0.11 g losses Eq [1]
Assume
“fermentable extract” = maltose, and that “losses” are settled biomass from
yeast synthesis (which I will represent as total suspended solids, TSS).
Balling’s
studies date from 1843 -1865, but his stoichiometry has stood up reasonably
well. It is established3,4 that Balling’s stoichiometry
somewhat under-predicts ethanol production; or, put another way, using it in
reverse to predict original extract (OE, original gravity in degrees Plato) for
a given %ABW tends to over-predict OE.
Several modern researchers3,4 have
raised theoretical objections to Balling’s stoichiometry — e.g., that
evaporations of water and alcohol are ignored; that net losses from yeast growth differ
significantly between lagers and ales;
that Balling's CO2 values are assumed (1 mol
CO2 per mol EtOH)
and not measured; and that not all CO2 produced will be evolved
(some will remain dissolved in the wort]).
Nonetheless, tinkering with Balling’s equation has ultimately resulted in
remarkably marginal improvements.
For example,
after lengthy, comprehensive analysis of large datasets, followed by derivation
of new equations, Cutaia et al. concluded,3
“Nonetheless, it is tribute to the analytical skills of Balling that he could
determine the Balling constants … in the mid-1800s. The original Balling values
are embedded in the brewing literature and their use … is reported to slightly
overestimate the original extract. Application of Balling’s original equation
to the datasets in this paper similarly overestimates original wort extract in all datasets but one. However, OE, RE and OHww measurement errors may overshadow
differences in the Balling constants. For these reasons and since the Balling
equation is embedded in the brewing literature (and culture!) we do not expect
(nor recommend) adaptation of a new Balling equation.”
Bioenergetics &
Stoichiometry: Another Approach
Yeast use maltose as both carbon source for synthesis of new
yeast cells, and as electron donor for energy production. Energy is required for synthesis of new cells
from maltose, and yeast derive that energy from
fermentation of maltose to EtOH under anoxic
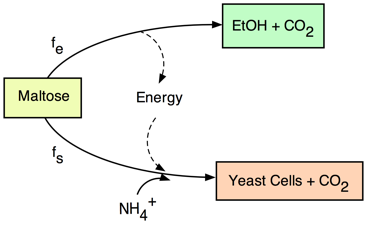
conditions. Thus, we can consider
that a fraction, fe,
of maltose is used for energy; and a fraction, fs, is
used for synthesis. Note that fs + fe
= 1.
Energy reaction
![]() Eq [2]
Eq [2]
Synthesis
reaction
To
write a synthesis reaction, converting maltose to yeast cells, we must have a
model for the composition of yeast.
Here we are presented with some difficulty: composition varies significantly with
species, strain, growth conditions, and stage of fermentation (i.e., early vs.
late).10,12
Simmonds,12 in averaging a number of
different sources, reported the organic portion (dry weight basis) of yeast is
about 90% of total dry weight, and in terms of major elements, the organic
portion is 48.3% C, 6% H, 34.5% O, and 10.6% N. This translates to a molar formula for
the major elements in the organic fraction of yeast of C4.03H6O2.16N0.76 (MW = 99.56 g/mol). Given the oxidation states of H (+I), O
(–II), and N(–III)[i.e., protein], such a
formula suggests that the average oxidation state of carbon is +0.149. It also means that in carbonaceous
oxidation of yeast to CO2 (producing +IV carbon), the number of electrons evolved would be:
4.03*(+4 - 0.149) = 15.52 for
C4.03H6O2.16N0.76. Thus, 1 electron equivalent (eeq) of yeast would be (1/15.52)*99.56 = 6.41 g VSS or
about 6.41/0.9 = 7.12 g TSS.
[Note: VSS = volatile suspended
solids, a standard measure of organic particular matter; TSS = total suspended
solids, a standard measure of total particulate matter (organic + ash).]
Rosen11(cited in Boulton and
Quain10) reported an elemental model for yeast VSS of C4.02H6.5O2.11N0.43P0.03. Let’s ignore the phosphorus
content, as being much smaller than the content of the other elements, giving C4.02H6.5O2.11N0.43
(MW = 94.52 g/mol). This implies the average oxidation state
of carbon is –0.246. [Note the more reduced state of carbon than if the empirical
formula of Simmonds is used, owing to the significantly lower nitrogen content
in Rosen’s formula.] In carbonaceous oxidation
to CO2 (+IV carbon), the number of electrons evolved would be: 4.02*(+4 + 0.246) = 17.07 for C4.02H6.5O2.11N0.43.
Thus, 1 eeq of yeast would be (1/17.07)*94.52 = 5.54
g VSS or about 5.54/0.9 = 6.16 g TSS.
An often-used9 empirical formula for the
elemental content of microbial biomass is C5H7O2N
(113 g/mol).
This wasn’t intended so much for yeast as for bacteria. In this formula, the average oxidation
state of carbon is zero – i.e., between those of the models of Simmonds
(+0.149) and Rosen (–0.246). The number of electrons evolved in carbonaceous
oxidation of C5H7O2N would be 20. Thus, 1 eeq
of C5H7O2N would be (1/20)*113 = 5.65 g VSS or
about 5.65/0.9 = 6.28 g TSS. This
is slightly higher than Rosen’s formula would predict (6.16 g TSS), but lower than
Simmonds’ (7.12 g TSS).
Conclusion: Given that yeast composition can
vary significantly depending upon strain and growing conditions, there is
little to recommend against the use of C5H7O2N as an empirical formula
for the ratio of major elements in the organic fraction of yeast. Though it was
formulated to model the principal elements in the organic fraction of bacterial
biomass, I am using it here to approximate the organic solids of yeast cells. It is an assumption positioned between
the sources10,11,12 consulted on yeast composition.
Thus,
the two half-reactions that are combined to produce a reaction for synthesis of
yeast cells from maltose are as follows:
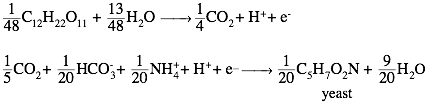
If we keep only the relevant terms and
normalize to one mole of maltose, the synthesis reaction becomes
![]() Eq [3]
Eq [3]
Yeast
cells (dry weight basis) are about 90% organic matter, 10% inorganic
matter. Since the formula weight of
C5H7O2N is 113 g/mol,
the 2.4 moles of C5H7O2N appearing on the
right-hand side of the synthesis reaction corresponds to 2.4(113)/0.9 = 301.3 g
total suspended solids (TSS).
Complete
Stoichiometry (combined energy and synthesis reactions)
The complete stoichiometry for maltose
fermentation is thus given by:
fe*Eq[2] +
(1 – fe)*Eq[3] Eq [4]
Our
quest, then, becomes estimation of fe, the net
fraction of maltose used for energy in fermentation of wort.
McCarty5-9
developed a method that uses
bioenergetics (based on thermodynamic data and observations about microbial
efficiencies of energy capture and utilization) to estimate cellular yields
from substrate degradation. With
estimates of specific decay/maintenance rate, the method allows fe to be estimated. For those readers who are familiar
with the method (e.g., students in my CEE6570 Biological Processes graduate class at
Cornell University), details are provided in the following. Readers unfamiliar with
the method will undoubtedly be lost; a tutorial is presented here.
∆G0(W), kcal
Rd: ![]() –10.0
–10.0
Ra: ![]() +7.592
+7.592
∆Gr
= 2.408 kcal
∆Gp = –1.455 kcal
![]()
![]()
![]()
Both
ae
and Y are gross yields (albeit
in different units), uncorrected for maintenance/decay, which should be
appreciable in prolonged fermentation.
Without such corrections, ae is the same as fs, the fraction of
malt used in biosynthesis; this means, uncorrected for
maintenance/decay, bioenergetics predicts fe = 1 – fs =
0.82 (clearly too low).
A
rational approach to correct for maintenance/decay: Consider that most of the yeast growth
occurs in the first few days, followed by autolysis ("endogenous
decay") over an extended, batch fermentation period. As reasonable
values, let's choose a specific decay rate, b
= 0.02 d-1
and a fermentation period of 30 days. The basis for selecting the decay
rate is that 2% per day is typical for anaerobic processes at sub-ambient
temperatures. The basis for selecting a period of 30
days? That's a typical fermentation period. Obviously, both decay
rate and fermentation period would be temperature-dependent, but in opposite
directions (i.e., decay rate would increase with temperature, while fermenation period would decrease with temperature).
Thus, their product is relatively insensitive to temperature, and it's the
product bt that
appears in the relevant equation.
Considered as an extended batch process (t= 30 d, b = 0.02 d-1)
fs = ae exp(–bt) = 0.179 exp[–0.02(30)]
= 0.10
------>
fe
= 0.90
Its use with Eq
[4] gives the following stoichiometric relationship:
1 g maltose ---> 0.484 g EtOH + 0.494 g CO2 + 0.088 g TSS Eq [5]
Comparison With
Balling’s Equation
Expressed per g maltose fermented,
Balling’s equation, Eq [1], becomes
1 g maltose
---> 0.4839 g EtOH + 0.4629 g CO2 +
0.0532 g TSS Eq [6]
What value of fe
used in Eq [4] corresponds to Balling’s
observations? The following Table was
created for different assumed values of fe:
|
Per gram maltose
consumed |
|||
|
fe |
g EtOH |
g CO2 |
g TSS |
|
0.90 |
0.484 |
0.494 |
0.088 |
|
0.91 |
0.490 |
0.496 |
0.079 |
|
0.92 |
0.495 |
0.498 |
0.070 |
|
0.93 |
0.500 |
0.500 |
0.062 |
|
0.94 |
0.506 |
0.502 |
0.053 |
|
0.95 |
0.511 |
0.504 |
0.044 |
|
0.96 |
0.516 |
0.506 |
0.035 |
|
0.97 |
0.522 |
0.508 |
0.026 |
|
0.98 |
0.527 |
0.511 |
0.018 |
|
0.99 |
0.533 |
0.513 |
0.009 |
|
1.00 |
0.538 |
0.515 |
0.000 |
|
|
makes EtOH agree
with Balling |
||
|
|
makes TSS agree with Balling |
||
It can be seen that no single value of fe
will give agreement with Balling for all three products (EtOH,
CO2, and TSS). In fact,
no reasonable value of fe allows
agreement for CO2. Since
Balling’s CO2 value was clearly based on the assumption of equal moles EtOH and CO2
produced in fermentation (rather than being based on actual measurement), there
is no reason we should concern ourselves with fitting it. Because Balling ignored the net CO2
that will be produced in synthesis of cells from maltose (Eq[3]), Balling’s equation
is necessarily going to underestimate CO2 produced per gram
maltose. On the other hand, not all
CO2 produced will be evolved (lost) from the beer; some will remain
dissolved. Balling’s theoretical
underestimation of CO2 production might unintentionally correct
(somewhat) for this. Nonetheless,
Balling’s underestimation of wort losses (CO2
and settled yeast cells) could, in part, explain his stoichiometry’s tendency
to underestimate ethanol production (%ABW): if wort losses
are underestimated, then final gravity is overestimated, lowering estimated EtOH weight percentage because the denominator (total final
wort mass per 100 g initial wort
mass) has been overestimated.
Conclusion
So, what
value of fe
am I going to use? In the end, I
elected to use a value consistent with both the bioenergetics analysis and with Balling’s
EtOH observations, since predicting EtOH production is my main objective:
---------> fe = 0.90
This
is the value employed in my Brix-based, %ABV calculator. Its use gives the
following stoichiometric relationship:
1 g maltose ---> 0.484 g EtOH
+ 0.494 g CO2 + 0.088 g TSS Eq [5]
Eq [5]
differs from Balling’s, Eq [6], in the following manner: My stoichiometry predicts greater CO2
evolution than Balling’s, since he ignored CO2 evolved from cell
synthesis; and
my stoichiometry predicts greater cell synthesis. Since only 10% of maltose is used in
synthesis, these differences in stoichiometry are not very important. Furthermore, they only come into play in
estimating final wort mass through subtraction of
evolved CO2 and settled biomass solids from initial wort mass. Since most of the wort
mass is water, these stoichiometric differences in estimated losses of CO2
and biomass solids are not very important. It is, however, more theoretically
satisfying to make these changes:
there is no theoretical justification for ignoring CO2
evolved in synthesis. It is also satisfying that application of
bioenergetics can predict a value of fe in reasonable agreement with Balling's
observations. In all this, it should be noted that I am walking in
the footsteps of other researchers who have raised theoretical objections to
Balling, but who have, in the end, not improved significantly upon his work.3,4
References
& Resources
1. Balling,
Carl. J. N., Die Bierbrauerei. Verlag von Friedrich Temski: Prague, CHZ, 1865.
2. Balling,
Carl J. N. “Die Gärungschemie” I-IV, Prague 1845, “Die Gärungschemie” I-III. 2.
Aufl., Prague 1854, “Lehrbuch der Bierbrauerei” I-II, 3. Aufl. Prague 1865.
3. Cutaia, A. J., A-J Reid, and R. A. Speers. “Examination
of the relationships between original, real and apparent extracts, and alcohol
in pilot plant and commercially produced beers,” Journal of the Institute of Brewing, 115(4), 318-327 (2009).
4. Neilson,
H., A. G. Kristiansen, K. M. Krieger Larsen, and C. Erikstrom. “Balling’s formula - scrutiny of a
brewing dogma,” Brauwelt Int., 25, 90-93 (2007)
5. McCarty,
P. L., “Energetics and Bacterial Growth,” in
S. D. Faust and J. V. Hunter (ed.), Organic
Compounds in Aquatic Environments, Marcel Dekker, Inc., New
York, pp. 495-512 (1971).
6. McCarty,
P. L., “Stoichiometry of Biological Reactions,” Progr. Water Technol.,
7,
157-172 (1975).
7. McCarty,
P. L., “Energetics of Organic Matter Degradation,” chapter 5 in Mitchell, R.
(ed.) Water Pollution Microbiology,
91-118, John Wiley & Sons (1972).
8. McCarty,
P. L., “Thermodynamics of Biological Synthesis and Growth,” The Proceedings of the 2nd International
Water Poll. Research. Conf., Tokyo,
1964. Pages 169-199, Pergamon Press (1965).
9. Rittmann, B. E., and P. L. McCarty, Environmental
Biotechnology: Principles and Applications, McGraw-Hill, New York, NY
(2001).
10. Boulton. C. and D. Quain, Brewing Yeast
& Fermentation. Oxford, U.
K., Blackwell Science Ltd. (2001).
11. Rosen, K. “Preparation of yeast for industrial use in the
production of beverages.” In Biotechnological
Applications in Beverage Production (eds C. Cantarelli and G. Lanzarini), pp.
164-223. Elsevier
Science Publishers, New York (1989).
12. Simmonds,
C., Alcohol,
Its Production, Properties, Chemistry, And Industrial Applications. Macmillan & Co. (1919).
![]()
![]() Overview Contribution of EtOH to Brix; Measurement of %ABV
Overview Contribution of EtOH to Brix; Measurement of %ABV ![]()